*Note: These notes were used as part of my biology, and are meant to provide an overview of organic chemistry and biochemistry at a 10th grade level.
1. Explain the structure of an atom.
The nucleus is the center of the atom. In the nucleus are protons (+) and neutrons (no charge). Electrons (-) revolve in shells around the nucleus.
2. Differentiate between elements and compounds.
Elements are substances that cannot be broken down any further. Compounds are 2 atoms that are bonded together.
3. What are the two types of bonds? How do they differ?
The two types of bonds are ionic and covalent. Ionic bonding is the transfer of electrons between atoms, while covalent bonding is the sharing of electrons.
4. What is an ion?
An ion is a charged particle formed by an atom that has gained or lost electrons.
5. Why is water called a polar molecule?
Water is called a polar molecule because the H atoms are bonded to the O atom at an angle, so that there is a negative charge on the O atoms and a positive charge on the H atoms.
6. How do molecular and structural formulas different?
Molecular formulas represent compounds by saying what is in the compound. Structural formulas show how the atoms are bonded.
7. What is an organic compound?
An organic compound is a compound in which the body of the molecule consists of carbon atoms covalently bonded to one another.
8. List the 4 types of organic compounds. What elements are they composed of? What is the function of each in the body? What are the monomers of each type?
-Carbohydrates- composed of C, H, and O in a C1H2O1. The monomer of carbohydrates is a saccharine. In the body, glucose is found in all cells,
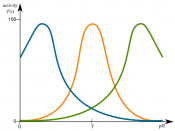
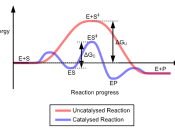
Comment
Great notes, really helped me with some revision this week. WELL DONE
1 out of 2 people found this comment useful.